what zinc oxide does to the bacteria and fungus to kill it
Abstract—
The search for effective and nontoxic sterilization drugs for plants against common phytopathogenic microorganisms is a major claiming to improve the biotechnology of plant clonal micropropagation. An analysis of 92 studies that depict the potential use of ZnO and TiO2 nanoparticles equally antimicrobial agents in biotechnology showed that their biological furnishings depend on several factors: photocatalytic activeness, particle size, concentration, morphology, and surface modification. The mechanisms of toxicity, among which the main 1 is generation of reactive oxygen species leading to oxidative stress, are likewise due to these factors. The data describing the straight influence of ZnO and TiO2 nanoparticles on plants, withal, are contradictory, which is probably because of the various particle shapes and sizes, their concentrations, and the species characteristics of the plants studied. These studies have confirmed that photocatalytically agile ZnO and TiO2 nanoparticles may be used every bit bactericidal and fungicidal drugs for sterilization of explants during clonal micropropagation of plants, while taking into business relationship the possible phytotoxicity of these particles.
INTRODUCTION
One of the main problems that arise during grooming of planting material is phytopathologies caused past diverse microorganisms: fungi and, less often, bacteria together with viruses. Microbial contamination is a serious threat, peculiarly for plant tissue civilisation, because information technology tin destroy explants. The organs obtained from plants under field conditions or in a greenhouse have previously undergone surface sterilization prior to introduce into the culture. The disinfection of explants is an of import footstep before in vitro cultivation, considering microorganisms in the growth medium grow faster than explants and tin seriously touch the results of microclonation. Sterilization, however, oftentimes seems to be rather ineffective. Disinfectants (bromine water, calcium hypochlorite, ethanol, hydrogen peroxide, sodium hypochlorite, mercuric chloride, silverish nitrate, antibiotics, and fungicides) are conventionally used to obtain sterile explants, but an increment in the concentration of disinfectants and the fourth dimension required for sterilization negatively bear upon the quality and viability of explants. In addition, some substances are phytotoxic [i, 2].
Nanoparticles and nanomaterials are currently considered to exist "new antibiotics." In particular, zinc oxide and titanium dioxide nanoparticles [3, 4] (Figs. ane, 2) are promising antimicrobial agents, because they possess photocatalytic activity, high penetration, and relative safety for multicellular organisms, at to the lowest degree in comparison with many other means for sterilization of explants.

Microphotographs of ZnO nanoparticles: (a) SEM and (b) TEM [3].

TEM images of TiO2 nanoparticles: (a) anatase and (b) rutile [4].
Information technology should exist noted that nanoparticles may too be used in the preparation of various sensors, herbicides, phytoimmunity stimulants, and agents for pesticide removal from plants and soil, in addition to the development of nanopesticides (Fig. 3) [5].
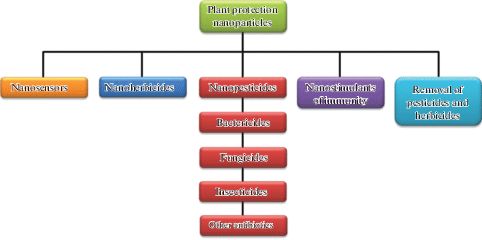
(Colour online) Directions for the utilise of nanoparticles in institute protection.
Antibacterial Properties of ZnO and TiO2 Nanoparticles
Nanosized zinc oxide can possess loftier antibacterial activity against various leaner and fungi, and a pregnant number of works confirms this fact [6–13]. The bactericidal backdrop of zinc oxide are currently being studied in both the macro- and nanoforms. The authors accept shown that ZnO has greater antimicrobial action when the particle size decreases to the nanometer range, because ZnO nanoparticles tin interact with the cell surface and/or nucleus during penetration into the cell [ix].
Although the biocidal activity of ZnO has been studied quite well, the exact machinery of toxicity is not fully explained and is controversial. The main possible machinery discussed in the literature is the following: straight contact of zinc oxide nanoparticles with the cell walls, leading to the destruction of membranes [six, 13–15], the release of antimicrobial ions (mainly Znii+), and the formation of reactive oxygen species [16, 17].
ZnO possesses the highest photocatalytic activity among all inorganic photocatalytic materials [18]. ZnO can effectively absorb UV radiations [nineteen], and, therefore, its photoactivity increases; this feature significantly enhances the interaction between ZnO and leaner. The activity of ZnO remains unchanged even afterward UV radiation is turned off, which is due to the electron depletion region attributable to negative oxygen atoms (O–ii and \({\text{O}}_{ii}^{{ - 2}}\)) adsorbed on the surface [20]. Zinc oxide nanoparticles in an aqueous solution under UV radiation have a phototoxic effect based on the product of H2O2 and O2– reactive oxygen species. A detailed reaction mechanism explaining this phenomenon was proposed before (Fig. iv) [11, 21, 22].
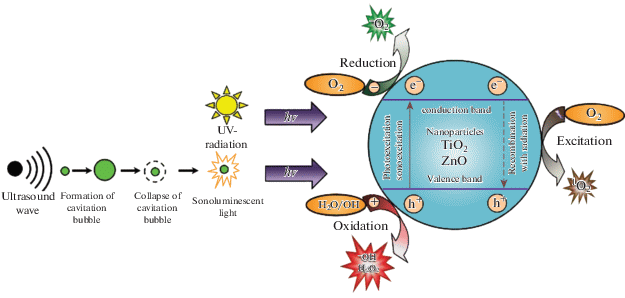
(Color online) Mechanisms for the generation of reactive oxygen species [22].
A higher antibacterial upshot of zinc oxide nanoparticles was detected later UV irradiation against Escherichia coli and Staphylococcus aureus (98.65 and 99.45%, respectively) [23]. ZnO, withal, possesses pregnant activity against bacteria under various test conditions (conventional lighting and in the night) [7, 24].
Many studies have shown that the various morphological parameters of ZnO nanoparticles, which are due to the synthesis atmospheric condition, influence the toxicity significantly [25, 27]. Desirable characteristics tin be achieved by variation of the following parameters: the solvent, forerunner, temperature, pH [28], and agents that regulate the class.
The antibacterial issue of ZnO nanoparticles in three different forms (nanorods, nanoflakes, and nanospheres) impregnated into depression density polyethylene (LDPE) confronting South. aureus ATCC 25923 was studied [29]. An analysis performed co-ordinate to ASTM E-2149 showed that ZnO nanospheres had the greatest inhibition of S. aureus. The shape of ZnO nanostructures tin affect their internalization machinery, because nanorods and nanowires can more hands penetrate bacterial cell walls than spherical nanoparticles [thirty]. At the aforementioned time, flower-shaped nanoparticles accept a college efficiency against South. aureus and E. coli than spherical and rod-shaped ones [31]. It was suggested that the polar faces of ZnO contribute to biochemical activity in addition to enhancement of internalization of zinc oxide nanoparticles through variation of the shape. In other words, more polar surfaces have a higher amount of oxygen vacancies. It is known that oxygen vacancies increase the generation of reactive oxygen species and, therefore, touch on the photocatalytic activity of ZnO [31].
The antibacterial activity of nanoparticles correlates straight with their concentration and depends on the size of particles. A big surface area and a higher concentration enhance the antibacterial effect of ZnO nanoparticles [fourteen, 32]. Smaller particles can easily penetrate bacterial membranes. The influence of the size (100–800 nm) of ZnO particles on their properties against Due south. aureus and East. coli was studied [33]. The authors institute that antibacterial action increases with a decrease in particle size. Similar effects were observed in other studies [vii, 11, fourteen].
The size-dependent bactericidal activity was assessed [12]. The authors analyzed the reaction of a number of gram-negative and gram-positive strains. They found that the antibacterial action of ZnO nanoparticles is inversely proportional to the particle size. An assay of the growth and viability curves of bacteria showed that the activity of nanoparticles depends on the size; i.e., smaller particles accept a greater antimicrobial upshot under visible calorie-free. These data indicate that ZnO nanoparticles with a very modest size (~12 nm) inhibited virtually 95% growth compared to that of the control. In addition, the influence of particles of diverse sizes (307, 212, 142, 88, and thirty nm) on bacterial growth at a concentration of 6 mmol was studied. The amount of viable cells decreased significantly with a decrease in particle size, which is due to the college reactivity of small nanoparticles [11].
The authors also institute that the antibacterial activity depends on the concentration and crystal construction of ZnO [34]. When the concentration was increased, bacterial survival decreased. A possible mechanism of toxicity, as the authors assumed, is violation of mitochondrial function, leakage of lactate dehydrogenase, and a change in the cell morphology under the activity of nanoparticles.
The influence of the dispersion medium and the storage time of suspensions of zinc oxide nanoparticles on their antibacterial activeness against the E. coli luminescent strain was studied [35]. The authors constitute that freshly prepared aqueous dispersions of nanoparticles at concentrations of 1, 10, 100, and k mg/L had the maximum activity: the survival rate was less than 5%; when the concentration decreased to 0.001 mg/50, the survival rate increased to 25%. Subsequently ane day, the survival rate remained unchanged only at high concentrations (one, x, 100, and m mg/50), whereas it was 80–90% at lower concentrations. When the aqueous environment was replaced with physiological saline (0.9% NaCl), the survival rate was less than 5% only at ten, 100, and 1000 mg/L, regardless of the storage time; when the concentration of nanoparticles was decreased, the biocidal event disappeared at 0.001 mg/L.
In that location is an electromagnetic allure between negatively charged bacteria and positively charged ZnO nanoparticles to class bonds between them. ZnO nanoparticles interact with membrane lipids and thiol groups (–SH) of enzymes and proteins, which are important for bacterial respiration, transmembrane transport, and intracellular ship. In addition, ZnO nanoparticles can penetrate into bacterial cells and inactivate phosphorus and sulfur compounds, such as Dna and enzymes. The generation of reactive oxygen species (ROSs) plays a central role in this process, because damage to membranes, Dna, and cellular proteins is due to ROSs [36]. This process is shown schematically in Fig. 5.
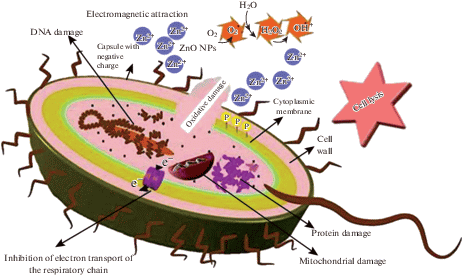
(Color online) The mechanism of antibacterial activeness of ZnO nanoparticles on S. typhi as an case [36].
Nanosized TiO2 is likewise effective in suppressing bacteria [21, 37–41].
Its antibacterial activity depends on the lite intensity [42], particle concentration and bore [43, 44], ambient temperature [45], substrate chemic composition [46, 47], and species sensitivity of microorganisms [48, 49].
The influence of ii TiO2 anatase types (25 and 100 nm) on the bacterial community in a filter with biologically activated carbon was assessed with Deoxyribonucleic acid analysis [50]. Both nanoparticle types significantly inhibited the level of bacterial adenosine triphosphate (ATP) (p < 0.01) and decreased the amount of copies of the 16S rDNA bacterial gene at 0.1 and 100 mg/Fifty. At the same time, the variety and uniformity of bacterial communities were significantly decreased. The relative amount of Nitrospira and Betaproteobacteria bacteria decreased later treatment with TiO2, whereas the amount of Bacilli and Gammaproteobacteria bacteria increased. The size of TiOtwo particles had a greater effect on the bacterial limerick than their concentration.
Hollow calcined nanospheres of titanium dioxide (CSTiO2), with a size of most 345 nm and a shell thickness of 17 nm and obtained by electrospinning and subsequent deposition onto the atomic layer, were studied [51]. The antibacterial action of CSTiO2 was assessed by the inhibition of growth of S. aureus (ATCC®6538TM control strain together with MRSA 97-vii and MRSA 622-4 resistant strains) and E. coli (ATCC®25922TM control strain and E. coli 33.1 resistant strain). Commercial titanium dioxide nanoparticles were used in the experiment for comparison. The studies showed that CSTiO2 had greater antibacterial activity confronting Due south. aureus and Due east. coli compared to that of commercial nanoparticles. At the same time, only CSTiOii had depression antibacterial activity confronting Due east. coli MRSA 33.1 in the study with resistant bacteria. The authors assume that such a low efficiency is probably due to the high resistance of bacteria to a wide range of exposure agents. UV radiations was used to enhance the antibacterial effect. After exposure for 60 min, the inhibitory effect of CSTiO2 at a concentration of 100 μg/mL confronting South. aureus MRSA 97-7 increased significantly; no similar effect was observed for TiO2 nanoparticles.
Fungicidal Action of ZnO and TiO2 Nanoparticles
Numerous studies indicate that zinc oxide nanoparticles possess a fungicidal effect. Indeed, ZnO nanoparticles obtained by the sol–gel method with a 0.15 and 0.i M precursor (zinc acetate dihydrate) inhibited the mycelial growth of the fungus Erythricium salmonicolor [52]. The inhibitory effect on fungal growth was studied past measuring the growth surface area every bit a part of time. Morphological changes were observed with high resolution optical microscopy (HROM), whereas transmission electron microscopy (TEM) was used to monitor changes in the ultrastructure. The results showed that the sample with a concentration of nine mmol/Fifty obtained from 0.fifteen M and 12 mmol/50 for the 0.1 M organisation significantly inhibited the growth of Eastward. salmonicolor. HROM images showed that there was a deformation in the growth structure: a noticeable thinning of hyphal fibers and a tendency to thicken. TEM results showed a liquefaction of the cytoplasmic contents, a decrease in its electron density in the presence of vacuoles, and significant damage to the cell wall.
The authors assessed the furnishings of ZnO nanoparticles on the viability of the pathogenic yeast Candida albicans [53]. They found that the outcome of ZnO on the viability of C. albicans depends on the concentration. They also found that the minimum fungicidal concentration of ZnO is 0.one mg/mL; information technology inhibited more than than 95% of C. albicans growth. ZnO nanoparticles as well inhibited the growth of C. albicans when added to the logarithmic phase of growth. The add-on of histidine (an inactivator of hydroxyl radicals and singlet oxygen) decreased the consequence of ZnO on C. albicans depending on the concentration. The antimycotic effect was nearly completely eliminated after adding five mmol of histidine. The excitation of ZnO with visible light increased the death of yeast cells. These furnishings of histidine imply that ROSs play a meaning role, including hydroxyl radicals and singlet oxygen, in cell death.
The antifungal action of zinc oxide nanoparticles with a size of 70 ± 15 nm at concentrations of 0, three, 6, and 12 mmol/L and the mechanism of their action against two pathogenic fungi (Botrytis cinerea and Penicillium expansum) were studied [54]. The results showed that ZnO nanoparticles at concentrations of more than three mmol/L tin significantly inhibit the growth of B. cinerea and P. expansum. P. expansum was more sensitive to ZnO treatment than B. cinerea. Scanning electron microscopy (SEM) and Raman spectroscopy data showed that there are 2 unlike antifungal mechanisms of ZnO against B. cinerea and P. expansum (Figs. half dozen, 7). ZnO nanoparticles inhibited the growth of B. cinerea, affecting cellular functions, which led to the deformation of fungal hyphae. In the case of P. expansum, ZnO nanoparticles prevented the growth of conidiophores and conidia, which ultimately led to the death of hyphae [54].
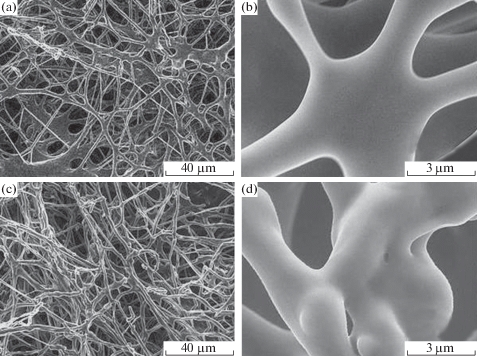
SEM images of Botrytis cinerea: (a, b) control and (c, d) afterward handling with ZnO [54].
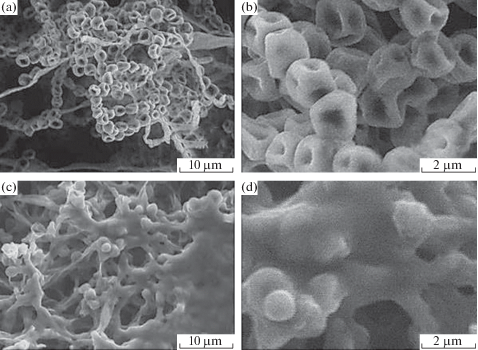
SEM images of Penicillium expansum: (a, b) control and (c, d) afterwards treatment with ZnO [54].
The antifungal activity of ZnO nanoparticles obtained under diverse synthesis conditions was assessed confronting Colletotrichum gloeosporioides strains [55]. In vitro activeness was establish past calculation of the minimum inhibitory concentrations (MICs). A articulate fungicidal effect was observed against ii C. gloeosporioides strains that had led to anthracnose in avocados and papayas. The MICs to suppress the pathogen isolated from papaya were 0.156 and 0.312 mg/mL for the avocado fungus, regardless of the method to prepare the nanomaterial. The inhibition of radial growth of the mycelium in the presence of nanoparticles was lx, 70, and 80% at concentrations of 0.156, 0.312, and 0.624 mg/mL, respectively.
ZnO nanoparticles inhibit the growth of Penicillium expansum at 0.5 mmol; the fungicidal effect intensified with an increase in concentration, and the pathogen was nearly completely suppressed at 15 mmol [56].
The first studies to assess the effectiveness of TiO2 against fungi appeared in 1985 [57]. They proved that the amount of Saccharomyces cerevisiae cells inactivated in vitro after 240 min of UV-A irradiation increased from 72 to 98% in the presence of a 0.five% colloidal solution of TiO2 nanoparticles. In improver, TiOtwo nanoparticles did not penetrate S. cerevisiae cells even afterward prolonged exposure, despite an increase in the ROS concentration in the cytosol [58]. These results indicate that the cell wall and plasma membrane were damaged insignificantly. The fungicidal properties of nanosized TiO2 were also observed against other C. albicans fungi [21, 40, 59–62]. Moreover, recent studies showed that TiO2 nanoparticles tin can be used to inactivate the mold species Fusarium sp. [41, 63], Aspergillus niger [21, 60, 64], and Penicillium expansum [65]. The relative resistance of fungi to photocatalytic oxidation is probably due to the protective result of polysaccharides in the cell wall [66].
The effect of titanium dioxide nanoparticles on Hypocrea lixii (white rot) and Mucor circinelloides (chocolate-brown rot), which are responsible for the rapid decay of forest, was studied [67]. The results showed that the photocatalytic activity of titanium dioxide nanoparticles prevents the fungal colonization of wood treated with suspensions of nanoparticles for a long time compared to untreated wood (Fig. 8).
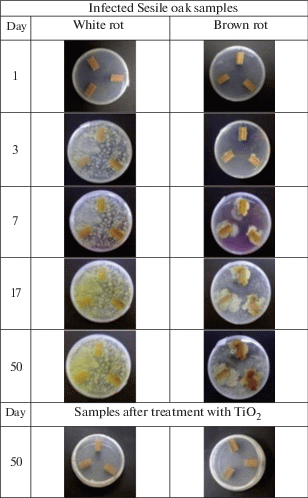
(Colour online) Mushroom growth on untreated and treated TiO2 samples of Sessile oak [67].
Influence of ZnO and TiO2 Nanoparticles on Plants
When nanoparticles are used as agents to sterilize explants, an important point is the assay of their effect on plants. Zinc is an of import essential element involved in many physiological processes in plants [68]. Information technology is an integral component of special proteins (zinc fingers), which bind to Dna and RNA and contribute to their regulation and stabilization [69]. Zinc is an integral part of diverse enzymes, for case, oxidoreductases, transferase, and hydrolases [70], likewise as ribosomes [71]. It plays an important office in the formation of carbohydrates and chlorophyll and for the growth of plant roots [72].
At the aforementioned time, zinc oxide nanoparticles can negatively consequence plant organisms. Indeed, the germination of corn seeds decreased under the influence of 2000 mg/Fifty of ZnO nanoparticles [73]. The length of the roots and stems of wheat decreased by 35 and 30% under the activity of zinc oxide nanoparticles at a concentration of 1000 mg/L, whereas the same parameters for cucumber plants decreased by 65 and 25%, respectively [74]. The biomass of buckwheat plants (Fagopyrum esculentum) decreased, and root cells were damaged under the action of 10–2000 mg/50 of zinc oxide in the substrate [75].
Treatment with ZnO dispersion nanoparticles significantly inhibited the growth of tomato plant roots and shoots: the biomass decreased by about ten% after treatment of 400 mg/dmthree of a substrate with ZnO and by 50% of plants treated with 800 mg/dm3. The corporeality of chlorophylls a and b and the efficiency of photosynthesis also decreased. The authors assumed that toxicity was probably because of damage to the photochemical system, which express photosynthesis and decreased biomass accumulation. ZnO nanoparticles too enhanced the transcription of genes of the antioxidant organization, which is probably due to the fact that ZnO can raise the protective response by an increase in the action of antioxidant enzymes [76].
ZnO nanoparticles can touch on the germination capacity of eggplant seeds depending on the cultivation medium [77]. Indeed, when seeds were germinated in the Murashige–Skoog medium, germination was inhibited with an increment in the concentration of nanoparticles from 5 to 20 mg/L and it decreased by more than 50% relative to the control at the maximum concentration. At the same time, germination in a peat medium was 100% at a concentration of nanoparticles of 20 and 100 mg/kg; when the concentration decreased to 5 mg/kg, formation decreased past 20%. Similar effects were observed for biomass growth. It especially should be noted that the maximum increment in length (~+25%) and mass (~+l%) of the root was observed when the concentration of peat was 100 mg/kg.
Later treatment with k and 1200 ppm of zinc oxide, 100% formation of seeds of corn plants was observed, whereas only 60% of the seeds germinated in the command. An increase in the concentration of nanoparticles to 1600 ppm, however, led to a subtract in the parameter to 40%. The authors besides establish that when the concentration of ZnO was 1200 ppm, there was a maximum increase in the found biomass [78]. These results may exist used to create weather for better rooting of plants during clonal micropropagation and to transfer microclones from a test tube to soil conditions.
Onion plants treated with ZnO nanoparticles at a concentration of xx and 30 μg/mL showed better growth and bloomed 12–14 days earlier than control plants [79]. The plants treated had higher values for seeds per umbel, seed weights per umbel, and grand seeds. Similar results betoken that ZnO nanoparticles can accelerate plant vegetation and provide ameliorate planting textile.
The influence of ZnO nanoparticles on the biochemical parameters of safflower plants was studied [fourscore]. The results showed that the corporeality of malondialdehyde increased at all concentrations of zinc oxide (10, 100, 500, and one thousand mg/L), which is probably due to the activation of complimentary radical reactions in the cells. The amount of guaiacol peroxidase, polyphenol oxidase, and dehydrogenase increased at concentrations of 100, ten, 500, and 1000 mg/L, respectively; in addition, the amount of dehydrogenase decreased at other concentrations. These data indicate that antioxidant systems are activated in the presence of nanoparticles, which is probably due to stress for plants.
The growth characteristics, the activity of photosynthesis, and biomass of wheat plants increased proportionally to the number of nanoparticles after treatment with ZnO nanoparticles at concentrations of 25, fifty, 75, and 100 mg/Fifty (Fig. nine) [81]. An assay of zinc accumulation showed that its concentration likewise increased linearly compared to the control: by 25, 43, 51, and 65% in the shoots; past twenty, 21, 29, and 43% in roots; and past 8, 35, l, and 64% in grains [81].
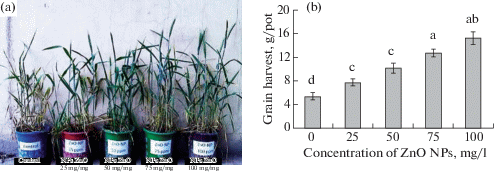
(Color online) The influence of ZnO nanoparticles on wheat at concentrations of 25, 50, 75, and 100 mg/L [81].
Treatment with zinc oxide nanoparticles increased the charge per unit of germination of capsicum seeds (Capsicum annuum 50.) during the offset seven days [82]. Germination increased past 12.50, 129.40, and 94.17% later treatment with ZnO suspensions of 100, 200, and 500 ppm, respectively. Analysis of the morphological parameters showed that treatment with nanoparticles did not take a significant effect on the development of the feather, simply affected significantly (p ≤ 0.01) the root length. The suspensions of nanoparticles (100, 200, and 500 ppm) inhibited the growth of roots and contributed to the accumulation of phenolic compounds in these organs.
The authors assessed the influence of various zinc compounds on the physiological reactions of habanero pepper plants (Capsicum chinense Jacq.) under greenhouse atmospheric condition [83]. They plant that ZnO nanoparticles at a concentration of 1000 mg/L had a positive effect on plant height, stem bore, and the amount of chlorophyll; it also increased the yield and biomass accumulation compared to that sample treated with ZnSO4. Zinc oxide at a concentration of 2000 mg/50 negatively affected the growth of plants, but significantly improved the quality of the fruit: the amount of capsaicin and dihydrocapsaicin increased by 19.3 and x.9%, respectively; the Scoville Heat Units (SHUs) increased by sixteen.4%. In improver, ZnO nanoparticles at 2000 mg/L also increased the amount of total phenols and total flavonoids (soluble + bound) in fruits (14.50 and 26.9%, respectively).
The influence of titanium dioxide in macroforms and nanoforms on seed germination, morphometric parameters of seedlings, and photosynthetic pigments of peppermint (Mentha piperita) was studied [84]. The authors showed that titanium dioxide samples at concentrations of 100, 200, and 300 mg/L inhibited seed formation. The evolution of seedlings was also suppressed; an exception was the case when the concentration of TiOtwo nanoparticles was 100 mg/Fifty, which led to an increase in the root length relative to the control. The amount of chlorophyll a and b increased under the action of TiO2 nanoparticles by more than ii times, regardless of the concentration. Macroform titanium dioxide had a positive effect only at 200 mg/50. The corporeality of carotenoids increased more than than two times relative to the control after handling with 100 mg/L TiOtwo, whereas macroform titanium dioxide at 200 mg/L increased this parameter by more than three times.
The influence of TiOtwo nanoparticles on the product and quality of rosemary essential oil (Rosmarinus officinalis) was assessed [85]. The experimental handling included the sputtering of TiO2 nanoparticles in concentrations of twenty, 40, lx, 100, 200, and 400 ppm on rosemary leaves. The results showed that the amount of many compounds in the essential oil with TiOtwo nanoparticles increased. This indicator, however, decreased at high concentrations (more than 200 ppm). An analysis of the corporeality of α-pinene, caryophyllene, and other compounds in the essential oil showed that information technology increased as much as possible, when the concentration of TiOtwo nanoparticles was 200 ppm.
The treatment of tomato seeds with suspensions of titanium dioxide nanoparticles (25 nm) at a concentration of k mg/Fifty significantly decreased the germination energy [86]. At the same time, there was no effect later the treatment of tomato plant seeds with TiOii dispersive nanoparticles (27 nm) at concentrations up to 4000 mg/L [87].
TiO2 nanoparticles also inhibited the rate of seed germination of maize and Narbonne peas [88]. The seed germination of soft wheat plants decreased in the presence of anatase titanium dioxide at a concentration of 150 mg/Fifty, whereas no such consequence was observed, when anatase and rutile were mixed [89].
The authors found that TiO2 anatase nanoparticles of most three nm in size penetrated into Arabidopsis thaliana cells and accumulated in vacuoles and nuclei of root cells and vacuoles or structures similar to endosomes in hypocotyl, cotyledon, and leaf cells [90]. Although this internalization of TiO2 nanoparticles did not affect the jail cell viability and morphology, the authors assumed that the absorption and distribution of nanoparticles lead to cellular and molecular changes. Further studies showed that TiO2 ultrafine anatase nanoparticles led to reorganization and emptying of microtubules of Arabidopsis thaliana with subsequent loftier degradation of tubulin monomers depending on proteasome. TiO2 nanoparticles induce the isotropic growth of root cells similar any other microtubule-destroying agents [91].
Moreover, some authors showed that titanium dioxide nanoparticles had positive effect on plant growth. Indeed, TiO2 at a concentration of 10 mg/L accelerated the formation of wheat seeds past 34% and contributed to a significant improvement in plant growth [92].
CONCLUSIONS
This review showed that ZnO and TiOii nanoparticles can be used successfully as antimicrobial agents, and their biological effect depends on certain factors: photocatalytic activity, particle size, concentration, morphology, and surface modification (Table 1). The toxicity mechanisms, the primary one of which is the generation of reactive oxygen species leading to oxidative stress, are likewise due to these factors.
The data concerning the directly effect of ZnO and TiO2 nanoparticles on plants, however, are contradictory, which is probably due to the diverse particle shapes and sizes, their concentrations, and species characteristics of plants. Thus, the studies ostend that photocatalytically active ZnO and TiO2 nanoparticles may be used effectively as bactericidal and fungicidal drugs for sterilizing explants during clonal micropropagation of plants, merely taking into account the possible phytotoxicity of these particles, which requires further written report.
REFERENCES
-
Y. H. Qin, J. A. T. Da Silva, J. H. Bi, et al., "Response of in vitro strawberry to antibiotics," Institute Growth Regul. 65, 183 (2011).
-
East. 5. Tambarussi, Grand. Rogalski, F. T. Southward. Nogueira, et al., "Influence of antibiotics on indirect organogenesis of teak," Ann. Forest Res. 58, 177 (2015).
-
J. Xia, K. Diao, Zh. Zheng, et al., "Porous Au/ZnO nanoparticles synthesised through a metal organic framework (MOF) route for enhanced acetone gas-sensing," RSC Adv. 7, 38444 (2017).
-
Ch. Uboldi, P. Urban, D. Gilliland, et al., "Office of the crystalline form of titanium dioxide nanoparticles: rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts," Toxicol. Vitro 31, 137 (2016).
-
Y. Shang, Grand. Grand. Hasan, 1000. J. Ahammed, et al., "Applications of nanotechnology in plant growth and crop protection: a review," Molecules 24, 2558 (2019).
-
R. Brayner, R. Ferrari-Iliou, N. Brivois, et al., "Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium," Nano Lett. half dozen, 866 (2006).
-
N. Jones, B. Ray, Thou. T. Ranjit, et al., "Antibacterial action of ZnO nanoparticle suspensions on a broad spectrum of microorganisms," FEMS Microbiol. Lett. 279, 71 (2008).
-
R. Jalal, Eastward. One thousand. Goharshadi, M. Abareshi, et al., "ZnO nanofluids: green synthesis, label, and antibacterial action," Mater. Chem. Phys. 121, 198 (2010).
-
J. T. Seil and T. J. Webster, "Antimicrobial applications of nanotechnology: methods and literature," Int. J. Nanomed. 7, 2767 (2012).
-
Z. Emami-Karvani and P. Chehrazi, "Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria," Afr. J. Microbiol. Res. 5, 1368 (2011).
-
Due north. Padmavathy and R. Vijayaraghavan, "Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study," Sci. Technol. Adv. Mater. ix, 035004 (2008).
-
K. R. Raghupathi, R. T. Koodali, and A. C. Manna, "Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles," Langmuir 27, 4020 (2011).
-
K. Kasemets, A. Ivask, H. C. Dubourguier, et al., "Toxicity of nanoparticles of ZnO, CuO, and TiO2 to yeast saccharomyces cerevisiae," Toxicol. Vitro 23, 1116 (2009).
-
L. Zhang, Y. Jiang, Y. Ding, et al., "Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids)," J. Nanopart. Res. 9, 479 (2007).
-
50. K. Adams, D. Y. Lyon, and P. J. Alvarez, "Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions," H2o Res. twoscore, 3527 (2006).
-
J. Sawai, S. Shoji, H. Igarashi, et al., "Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry," J. Biosci. Bioeng. 86, 521 (1998).
-
50. Zhang, Y. Ding, K. Povey, et al., "ZnO nanofluids-a potential antibacterial agent," Prog. Nat. Sci.: Mater. Int. eighteen, 939 (2008).
-
J. Zhang, "Silver-coated zinc oxide nanoantibacterial synthesis and antibacterial activity characterization," in Proceedings of the 2011 International Conference on Electronics and Optoelectronics (ICEOE), Dalian, Liaoning, United states, July 29–31, 2011, Vol. 3, p. 94. https://doi.org/x.1109/ICEOE.2011.6013309
-
M. Nirmala, M. G. Nair, Chiliad. Rekha, et al., "Photocatalytic activity of ZnO nanopowders synthesized by DC thermal plasma," Afr. J. Basic Appl. Sci. 2, 161 (2010).
-
Proceedings of the Photoconductivity Conference, Atlantic City, Nov. 4–6, 1954, Ed. by R. G. Breckenridge, B. R. Russell, and E. E. Hahn (Wiley, New York, 1956).
-
O. Seven, B. Dindar, Southward. Aydemir, et al., "Solar photocatalytic disinfection of a grouping of leaner and fungi aqueous suspensions with TiO2, ZnO, and Sahara Desert grit," J. Photochem. Photobiol. A 165, 103 (2004).
-
J. Bogdan, J. Pławińska-Czarnak, and J. Zarzyńska, "Nanoparticles of titanium and zinc oxides as novel agents in tumor treatment: a review," Nanoscale Res. Lett. 12, 225 (2017).
-
Grand. Zhou, Y. Li, West. Xiao, et al., "Synthesis, characterization, and antibacterial activities of a novel nanohydroxyapatite/zinc oxide circuitous," J. Biomed. Mater. Res. A 85, 929 (2008).
-
P. Joshi, S. Chakraborti, P. Chakrabarti, et al., "Role of surface adsorbed anionic species in antibacterial activeness of ZnO quantum dots against escherichia coli," J. Nanosci. Nanotechnol. ix, 6427 (2009).
-
A. Stanković, Southward. Dimitrijević, and D. Uskoković, "Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothermally synthesized using different surface stabilizing agents," Colloids Surf., B 102, 21 (2013).
-
Due north. Talebian, S. M. Amininezhad, and Yard. Doudi, "Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical backdrop," J. Photochem. Photobiol. A 120, 66 (2013).
-
J. Ma, J. Liu, Y. Bao, et al., "Synthesis of large-scale compatible mulberry-like ZnO particles with microwave hydrothermal method and its antibacterial holding," Ceram. Int. 39, 2803 (2013).
-
P. J. P. Espitia, Northward. F. F. Soares, J. South. R. Coimbra, et al., "Zinc oxide nanoparticles: synthesis, antimicrobial action, and food packaging applications," Food Bioprocess Technol. 5, 1447 (2012).
-
North. H. Harun R. B. S. One thousand. N. Mydin, Due south. Southward. Sreekantan, et al., "Shape-dependent antibacterial activity against staphylococcus aureus of zinc oxide nanoparticles," Malays. J. Med. Wellness Sci. 14, 141 (2018).
-
H. Yang, C. Liu, D. Yang, et al., "Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the part of particle size, shape and composition," J. Appl. Toxicol. 29, 69 (2009).
-
1000. Li, T. Hu, 1000. Pan, et al., "Morphology-function human relationship of ZnO: polar planes, oxygen vacancies, and activeness," J. Phys. Chem. C 112, 11859 (2008).
-
10. Peng, S. Palma, N. S. Fisher, et al., "Issue of morphology of ZnO nanostructures on their toxicity to Marine algae," Aquatic Toxicol. 102, 186 (2011).
-
O. Yamamoto, "Influence of particle size on the antibacterial activity of zinc oxide," Int. J. Inorg. Mater. 3, 643 (2001).
-
H. A. Jeng and J. Swanson, "Toxicity of metal oxide nanoparticles in mammalian cells," J. Environ. Sci. Health A 41, 2699 (2006).
-
O. Zakharova, E. Kolesnikov, E. Vishnyakova, et al., "Antibacterial activity of ZnO nanoparticles: dependence on particle size, dispersion media and storage time," IOP Conf. Ser.: Globe Environ. Sci. 226, 012062 (2019).
-
R. Meraat, A. A. Ziabari, Kh. Issazadeh, et al., "Synthesis and characterization of the antibacterial activity of zinc oxide nanoparticles confronting Salmonella typhi," Acta Metall. Sin. (Engl. Lett.) 29, 601 (2016).
-
B. Kim, D. Kim, D. Cho, et al., "Bactericidal effect of TiO2 photocatalyst on selected nutrient-borne pathogenic bacteria," Chemosphere 52, 277 (2003).
-
A. Azam, A. Southward. Ahmed, M. Oves, et al., "Antimicrobial activity of metallic oxide nanoparticles confronting gram-positive and gramnegative bacteria: a comparative study," Int. J. Nanomed. 7, 6003 (2012).
-
K. Azimzadehirani, One thousand. Elahifard, S. Haghighi, et al., "Highly efficient hydroxyapatite/TiO2 composites covered by silver halides as E. coli disinfectant nether visible light and dark media," Photochem. Photobiol. Sci. 12, 1787 (2013).
-
J. Lonnen, L. J. Kilvington, S. C. Kehoe, et al., "Solar and photocatalytic disinfection of protozoan, fungal, and bacterial microbes in drinking water," Water Res. 39, 877 (2005).
-
C. Sichel, J. Tello, M. de Cara, et al., "Issue of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi," Catal. Today 129, 152 (2007).
-
A. K. Benabbou, Z. Derriche, C. Felix, et al., "Photocatalytic inactivation of escherichia coli-result of concentration of TiOtwo and microorganism, nature, and intensity of UV irradiation," Appl. Catal. B 76, 257 (2007).
-
A. Simon-Deckers, Due south. Loo, M. Mayne-Fifty'Hermite, et al., "Size- composition- and shape-dependent toxicological bear upon of metal oxide nanoparticles and carbon nanotubes towards leaner," Environ. Sci. Technol. 43, 8423 (2009).
-
B. Li and B. E. Logan, "The impact of ultraviolet light on bacterial adhesion to drinking glass and metal oxide-coated surface," Colloids Surf. B 41, 153 (2005).
-
D. Friedmann, C. Mendive, and D. Bahnemann, "TiO2 for water treatment: parameters affecting the kinetics and mechanisms of photocatalysis," Appl. Catal. B 99, 98 (2010).
-
P. C. Maness, S. Smolinski, D. M. Blake, et al., "Bactericidal action of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism," Appl. Environ. Microbiol. 65, 4094 (1999).
-
T. Tong, C. T. Binh, J. J. Kelly, et al., "Cytotoxicity of commercial nano-TiO2 to Escherichia coli assessed by loftier-throughput screening: effects of environmental factors," Water Res. 47, 2352 (2013).
-
H. A. Foster, I. B. Ditta, S. Varghese, et al., "Photocatalytic disinfection using titanium dioxide: spectrum and machinery of antimicrobial activity," Appl. Microbiol. Biotechnol. ninety, 1847 (2011).
-
Y. H. Tsunag, J. Southward. Sun, Y. C. Huang, et al., "Studies of photokilling of bacteria using titanium dioxide nanoparticles," Artif. Organs 32, 167 (2008).
-
L. Zhiyuan, Y. Shuili, P. Heedeung, et al., "Bear upon of titanium dioxide nanoparticles on the bacterial coммoль unities of biological activated carbon filter intended for drinking h2o treatment," Environ. Sci. Pollut. Res. 23, 15574 (2016).
-
C. L. de Dicastillo, C. Patiño, Chiliad. J. Galotto, et al., "Novel hollow titanium dioxide nanospheres with antimicrobial activity confronting resistant leaner," Beilstein J. Nanotechnol. 19, 1716 (2019).
-
P. A. Arciniegas-Grijalba, Chiliad. C. Patiño-Portela, L. P. Mosquera-Sánchez, et al., "ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee mucus erythricium salmonicolor," Appl. Nanosci. 7, 225 (2017).
-
A. Lipovsky, Y. Nitzan, A. Gedanken, et al., "Antifungal activeness of ZnO nanoparticles—the role of ROS mediated cell injury," Nanotecnology 22, 105101 (2011).
-
50. He, Y. Liu, A. Fifty. Mustapha, et al., "Antifungal activity of zinc oxide nanoparticles confronting Botrytis cinerea and Penicillium expansum," Microbiol. Res. 166, 207 (2011).
-
S. C. de la Rosa-García, P. Martínez-Torres, S. Gómez-Cornelio, et al., "Antifungal activity of ZnO and MgO nanomaterials and their mixtures against colletotrichum gloeosporioides strains from tropical fruit," J. Nanomater., 3498527 (2018).
-
D. Sardell, R. Gatt, and V. P. Valdramidisa, "Assessing the efficacy of zinc oxide nanoparticles against penicillium expansum by automatic turbidimetric analysis," Mycology 9, 43 (2018).
-
T. Matsunaga, R. Tomoda, T. Nakajima, et al., "Photoelectrochemical sterilization of microbial cells by semiconductor powders," FEMS Microbiol. Lett. 29, 211 (1985).
-
South. Thabet, F. Simonet, M. Lemaire, et al., "Affect of photocatalysis on fungal cells: depiction of cellular and molecular effects on Saccharomyces cerevisiae," Appl. Environ. Microbiol. 80, 7527 (2014).
-
N. Akiba, I. Hayakawa, E. South. Keh, et al., "Antifungal effects of a tissue conditioner coating agent with TiO2 photocatalyst," J. Med. Dent. Sci. 52, 223 (2005).
-
D. Mitoraj, A. Jańczyk, M. Strus, et al., "Visible calorie-free inactivation of bacteria and fungi past modified titanium dioxide," Photochem. Photobiol. Sci. vi, 642 (2007).
-
I. Perelshtein, G. Applerot, North. Perkas, et al., "A one-pace process for the antimicrobial finishing of textiles with crystalline TiO2 nanoparticles," Chem.-Eur. J. 18, 4575 (2012).
-
G. Xiao, X. Zhang, Y. Zhao, et al., "The behavior of active bactericidal and antifungal coating under visible light irradiation," Appl. Surf. Sci. 292, 756 (2014).
-
C. Sichel, M. de Cara, J. Tello, et al., "Solar photocatalytic disinfection of agricultural pathogenic fungi: fusarium species," Appl. Surf. Sci. 74, 152 (2007).
-
M. P. Yu, Y. T. Huang, and S. C. Yang, "The antifungal efficacy of nano-metals supported TiO2 and ozone on the resistant aspergillus niger spore," J. Take chances. Mater. 261, 155 (2013).
-
Southward. Y. Ye, M. L. Fan, X. L. Song, et al., "Enhanced photocatalytic disinfection of p. expansum in cold storage using a TiO2/ACF motion picture," Int. J. Nutrient Microbiol. 136, 332 (2010).
-
E. Barreto-Bergter and R. T. Figueiredo, "Fungal glycans and the innate immol une recognition," Front. Jail cell. Infection Microbiol. 4, 145 (2014).
-
Thou. de Filpo, A. M. Palermo, F. Rachiele, et al., "Preventing fungal growth in forest past titanium dioxide nanoparticles," Int. Biodeterior. Biodegrad. 85, 217 (2013).
-
R. Sagardoy, F. Morales, A. F. Lopez-Millan, et al., "Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics," Plant Biol. 11, 339 (2009).
-
S. G. Gupta, A. K. Rai, S. Southward. Kanwar, et al., "Comparative analysis of zinc finger proteins involved in institute disease resistance," PLoS One vii (8), e42578 (2012).
-
Southward. Mishra and R. S. Dubey, "Heavy metal toxicity induced alterations in photosynthetic metabolism in plants," in Handbook of Photosynthesis, Ed. past M. Pessarakli, 2d ed. (CRC, Taylor and Francis, New York, 2005), p. 845.
-
S. R. Mousavi, M. Galavi, and M. Rezaei, "Zinc (Zn) importance for crop product—a review," Int. J. Agron. Institute Prod. 4, 64 (2013).
-
A. Kleckerova, P. Sobrova, and O. Krystofova, "Cadmium(2) and zinc(Ii) ions furnishings on maize plants revealed by spectroscopy and electrochemistry," Int. J. Electrochem. Sci. 6, 6011 (2011).
-
D. Lin and B. Xing, "Phytotoxicity of nanoparticles: inhibition of seed germination and root growth," Environ. Pollut. 150, 243 (2007).
-
S. Kumar, A. Thousand. Patra, Southward. C. Datta, et al., "Phytotoxicity of nanoparticles to seed germination of plants," Int. J. Adv. Res. 3, 854 (2015).
-
Southward. Lee, H. Chung, Due south. Kim, et al., "The genotoxic result of ZnO and CuO nanoparticles on early on growth of buckwheat, fagopyrum esculentum," Water Air Soil Pollut. 224, 1668 (2013).
-
X. P. Wang, Q. Q. Li, Z. Chiliad. Pei, et al., "Furnishings of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plant plants," Biol. Constitute. 62, 801 (2018).
-
T. Thunugunta, R. Channa, S. Kodthalu, et al., "Affect of zinc oxide nanoparticles on eggplant (S. melongena): studies on growth and the accumulation of nanoparticles," IET Nanobiotechnol. 12, 706 (2018).
-
D. S. Meena, H. M. Jayadeva, Ch. Gautam, et al., "Furnishings of nano zinc oxide (ZnO) particles on formation of Maize (Zea mays L.) seeds," Int. J. Plant Soil Sci. 16, 1 (2017).
-
Southward. V. Raskar and S. Fifty. Laware, "Influence of zinc oxide nanoparticles on growth, floweringand seed productivity in onion," Int. J. Curr. Microbiol. Appl. Sci. three, 874 (2014).
-
Z. Hafizi and N. Nasr, "The effect of zinc oxide nanoparticles on safflower plant growth and physiology," Eng., Technol. Appl. Sci. Res. 8, 508 (2018).
-
T. Munir, Thousand. Rizwan, M. Kashif, et al., "Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticumaestivum L.) by seed priming method," Digest J. Nanomater. Biostruct. 13, 315 (2018).
-
J. I. García-López, F. Zavala-García, E. Olivares-Sáenz, et al., "Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during formation," Agronomy 8 (10), 215 (2018).
-
J. I. García-López, K. Niño-Medina, E. Olivares-Sáenz, et al., "Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers," Plants 8 (viii), 254 (2019).
-
North. Samadi, Due south. Yahyaabadi, and Z. Rezayatmand, "Effect of TiO2 and TiO2 nanoparticle on germination, root, and shoot length and photosynthetic pigments of mentha piperita," Int. J. Institute Soil Sci. 3, 408 (2014).
-
A. Golami, H. Abbaspour, H. Hashemi-Moghaddam, and M. Gerami, "Photocatalytic event of TiOtwo nanoparticles on essential oil of Rosmarinus officinalis," J. Biochem. Technol. nine (four), fifty (2018).
-
R. Raliya, R. Nair, S. Chavalmane, et al., "Mechanistic evaluation of translocation and physiological touch of titanium dioxide and zinc oxide nanoparticles on the tomato plant (Solanum lycopersicum L.) plant," Metallomics vii, 1584 (2015).
-
U. Song, H. Jun, B. Waldman, et al., "Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiOtwo and Ag on tomatoes (Lycopersicon esculentum)," Ecotoxicol. Environ. Safe 93, 60 (2013).
-
M. Castiglione, 50. Giorgetti, C. Geri, et al., "The effects of nano-TiOtwo on seed germination, development, and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L.," J. Nanopart. Res. 13, 2443 (2011).
-
C. Larue, H. Khodja, N. Herlin-Boime, et al., "Investigation of titanium dioxide nanoparticles toxicity and uptake by plants," J. Phys.: Conf. Ser. 304, 012057 (2011).
-
J. Kurepa, T. Paunesku, S. Vogt, et al., "Uptake and distribution of ultrasmall anatase TiO2 alizarin Cherry-red S nanoconjugates in Arabidopsis thaliana," Nano Lett. 10, 2296 (2010).
-
South. Wang, J. Kurepa, and J. A. Smalle, "Ultra-pocket-sized TiO2 nanoparticles disrupt microtubular networks in arabidopsis thaliana," Plant, Cell Environ. 34, 811 (2011).
-
H. Feizi, P. Moghaddam, N. Shahtahmassebi, et al., "Affect of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth," Biolog. Trace Elem. Res. 146, 101 (2012).
Funding
This work was conducted nether a State Job of the Russian Ministry building of Science and Education (project no. 14. 13544.2019 13.ane).
Author information
Affiliations
Corresponding author
Additional data
Translated past A. Tulyabaev
Rights and permissions
Nearly this article
Cite this article
Zakharova, O.V., Gusev, A.A. Photocatalytically Active Zinc Oxide and Titanium Dioxide Nanoparticles in Clonal Micropropagation of Plants: Prospects. Nanotechnol Russia xiv, 311–324 (2019). https://doi.org/10.1134/S1995078019040141
-
Received:
-
Revised:
-
Accustomed:
-
Published:
-
Consequence Date:
-
DOI : https://doi.org/10.1134/S1995078019040141
Source: https://link.springer.com/article/10.1134/S1995078019040141
0 Response to "what zinc oxide does to the bacteria and fungus to kill it"
Post a Comment