What would happen to the supply of ATP in your cells if you did not eat enough carbohydrates
As we have only seen, cells crave a constant supply of energy to generate and maintain the biological order that keeps them alive. This energy is derived from the chemical bond energy in food molecules, which thereby serve as fuel for cells.
Sugars are particularly important fuel molecules, and they are oxidized in small steps to carbon dioxide (COtwo) and water (Figure two-69). In this section nosotros trace the major steps in the breakdown, or catabolism, of sugars and bear witness how they produce ATP, NADH, and other activated carrier molecules in animal cells. We concentrate on glucose breakup, since it dominates free energy product in most creature cells. A very similar pathway also operates in plants, fungi, and many bacteria. Other molecules, such as fatty acids and proteins, can also serve as free energy sources when they are funneled through appropriate enzymatic pathways.

Effigy 2-69
Schematic representation of the controlled stepwise oxidation of sugar in a cell, compared with ordinary called-for. (A) In the cell, enzymes catalyze oxidation via a series of small-scale steps in which free energy is transferred in conveniently sized packets (more...)
Nutrient Molecules Are Broken Down in Three Stages to Produce ATP
The proteins, lipids, and polysaccharides that make upwards nigh of the food we swallow must exist broken down into smaller molecules before our cells can use them—either as a source of energy or as edifice blocks for other molecules. The breakdown processes must act on food taken in from exterior, only non on the macromolecules within our own cells. Stage ane in the enzymatic breakdown of food molecules is therefore digestion, which occurs either in our intestine outside cells, or in a specialized organelle within cells, the lysosome. (A membrane that surrounds the lysosome keeps its digestive enzymes separated from the cytosol, equally described in Chapter 13.) In either example, the large polymeric molecules in food are broken downward during digestion into their monomer subunits—proteins into amino acids, polysaccharides into sugars, and fats into fatty acids and glycerol—through the action of enzymes. Afterward digestion, the small-scale organic molecules derived from food enter the cytosol of the cell, where their gradual oxidation begins. As illustrated in Figure 2-70, oxidation occurs in ii further stages of cellular catabolism: stage 2 starts in the cytosol and ends in the major energy-converting organelle, the mitochondrion; stage 3 is entirely confined to the mitochondrion.
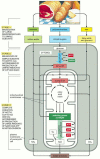
Figure 2-lxx
Simplified diagram of the 3 stages of cellular metabolism that lead from food to waste products in creature cells. This series of reactions produces ATP, which is and then used to drive biosynthetic reactions and other energy-requiring processes in the (more...)
In stage 2 a chain of reactions called glycolysis converts each molecule of glucose into two smaller molecules of pyruvate. Sugars other than glucose are similarly converted to pyruvate after their conversion to one of the carbohydrate intermediates in this glycolytic pathway. During pyruvate germination, 2 types of activated carrier molecules are produced—ATP and NADH. The pyruvate then passes from the cytosol into mitochondria. There, each pyruvate molecule is converted into COtwo plus a two-carbon acetyl grouping—which becomes attached to coenzyme A (CoA), forming acetyl CoA, another activated carrier molecule (see Figure 2-62). Large amounts of acetyl CoA are also produced past the stepwise breakup and oxidation of fatty acids derived from fats, which are carried in the bloodstream, imported into cells equally fatty acids, and and then moved into mitochondria for acetyl CoA product.
Stage iii of the oxidative breakup of food molecules takes place entirely in mitochondria. The acetyl group in acetyl CoA is linked to coenzyme A through a loftier-energy linkage, and information technology is therefore hands transferable to other molecules. Afterwards its transfer to the four-carbon molecule oxaloacetate, the acetyl group enters a series of reactions called the citric acid cycle. As nosotros talk over shortly, the acetyl group is oxidized to CO2 in these reactions, and large amounts of the electron carrier NADH are generated. Finally, the high-energy electrons from NADH are passed along an electron-transport chain within the mitochondrial inner membrane, where the energy released by their transfer is used to drive a process that produces ATP and consumes molecular oxygen (O2). It is in these final steps that near of the energy released by oxidation is harnessed to produce about of the cell's ATP.
Because the energy to drive ATP synthesis in mitochondria ultimately derives from the oxidative breakdown of food molecules, the phosphorylation of ADP to form ATP that is driven by electron transport in the mitochondrion is known as oxidative phosphorylation. The fascinating events that occur within the mitochondrial inner membrane during oxidative phosphorylation are the major focus of Chapter fourteen.
Through the production of ATP, the energy derived from the breakdown of sugars and fats is redistributed as packets of chemical energy in a form user-friendly for apply elsewhere in the cell. Roughly tenix molecules of ATP are in solution in a typical cell at any instant, and in many cells, all this ATP is turned over (that is, used up and replaced) every 1–2 minutes.
In all, almost half of the free energy that could in theory be derived from the oxidation of glucose or fatty acids to H2O and CO2 is captured and used to bulldoze the energetically unfavorable reaction Pi + ADP → ATP. (Past dissimilarity, a typical combustion engine, such as a machine engine, can convert no more than 20% of the bachelor energy in its fuel into useful work.) The residue of the free energy is released by the cell as heat, making our bodies warm.
Glycolysis Is a Central ATP-producing Pathway
The most of import procedure in stage two of the breakup of nutrient molecules is the degradation of glucose in the sequence of reactions known as glycolysis—from the Greek glukus, "sweet," and lusis, "rupture." Glycolysis produces ATP without the interest of molecular oxygen (Otwo gas). Information technology occurs in the cytosol of most cells, including many anaerobic microorganisms (those that can alive without utilizing molecular oxygen). Glycolysis probably evolved early in the history of life, earlier the activities of photosynthetic organisms introduced oxygen into the atmosphere. During glycolysis, a glucose molecule with vi carbon atoms is converted into 2 molecules of pyruvate, each of which contains iii carbon atoms. For each molecule of glucose, two molecules of ATP are hydrolyzed to provide energy to drive the early steps, but four molecules of ATP are produced in the later steps. At the end of glycolysis, there is consequently a net gain of two molecules of ATP for each glucose molecule broken down.
The glycolytic pathway is presented in outline in Effigy 2-71, and in more than particular in Panel 2-viii (pp. 124–125). Glycolysis involves a sequence of 10 carve up reactions, each producing a different carbohydrate intermediate and each catalyzed past a different enzyme. Similar most enzymes, these enzymes all have names ending in ase—like isomerase and dehydrogenase—which signal the blazon of reaction they catalyze.
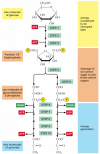
Figure 2-71
An outline of glycolysis. Each of the 10 steps shown is catalyzed past a different enzyme. Note that step iv cleaves a six-carbon sugar into ii three-carbon sugars, and then that the number of molecules at every stage after this doubles. As indicated, step half-dozen (more...)
![]()
Panel 2-eight
Details of the 10 Steps of Glycolysis.
Although no molecular oxygen is involved in glycolysis, oxidation occurs, in that electrons are removed past NAD+ (producing NADH) from some of the carbons derived from the glucose molecule. The stepwise nature of the procedure allows the energy of oxidation to exist released in modest packets, and then that much of it can be stored in activated carrier molecules rather than all of it beingness released as rut (see Figure 2-69). Thus, some of the energy released by oxidation drives the straight synthesis of ATP molecules from ADP and Pi, and some remains with the electrons in the high-free energy electron carrier NADH.
Two molecules of NADH are formed per molecule of glucose in the course of glycolysis. In aerobic organisms (those that require molecular oxygen to live), these NADH molecules donate their electrons to the electron-transport chain described in Affiliate 14, and the NAD+ formed from the NADH is used again for glycolysis (meet step 6 in Panel 2-viii, pp. 124–125).
Fermentations Allow ATP to Be Produced in the Absence of Oxygen
For most fauna and plant cells, glycolysis is only a prelude to the third and final stage of the breakdown of food molecules. In these cells, the pyruvate formed at the terminal step of stage 2 is rapidly transported into the mitochondria, where it is converted into COii plus acetyl CoA, which is so completely oxidized to COtwo and H2O.
In contrast, for many anaerobic organisms—which do not apply molecular oxygen and can grow and divide without it—glycolysis is the principal source of the prison cell's ATP. This is as well true for certain fauna tissues, such as skeletal musculus, that can proceed to role when molecular oxygen is limiting. In these anaerobic weather condition, the pyruvate and the NADH electrons stay in the cytosol. The pyruvate is converted into products excreted from the cell—for example, into ethanol and CO2 in the yeasts used in brewing and breadmaking, or into lactate in muscle. In this process, the NADH gives up its electrons and is converted back into NAD+. This regeneration of NAD+ is required to maintain the reactions of glycolysis (Figure 2-72).
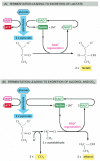
Figure 2-72
2 pathways for the anaerobic breakdown of pyruvate. (A) When inadequate oxygen is present, for instance, in a muscle cell undergoing vigorous contraction, the pyruvate produced by glycolysis is converted to lactate as shown. This reaction regenerates (more than...)
Anaerobic free energy-yielding pathways like these are called fermentations. Studies of the commercially important fermentations carried out by yeasts inspired much of early biochemistry. Work in the nineteenth century led in 1896 to the then startling recognition that these processes could be studied exterior living organisms, in cell extracts. This revolutionary discovery somewhen made information technology possible to dissect out and written report each of the individual reactions in the fermentation process. The piecing together of the consummate glycolytic pathway in the 1930s was a major triumph of biochemistry, and it was quickly followed by the recognition of the central role of ATP in cellular processes. Thus, near of the fundamental concepts discussed in this chapter take been understood for more 50 years.
Glycolysis Illustrates How Enzymes Couple Oxidation to Energy Storage
We take previously used a "paddle cycle" analogy to explain how cells harvest useful energy from the oxidation of organic molecules by using enzymes to couple an energetically unfavorable reaction to an energetically favorable 1 (meet Figure two-56). Enzymes play the part of the paddle wheel in our analogy, and we now return to a pace in glycolysis that nosotros have previously discussed, in guild to illustrate exactly how coupled reactions occur.
2 central reactions in glycolysis (steps vi and vii) convert the three-carbon sugar intermediate glyceraldehyde three-phosphate (an aldehyde) into 3-phosphoglycerate (a carboxylic acid). This entails the oxidation of an aldehyde group to a carboxylic acid group, which occurs in two steps. The overall reaction releases plenty free energy to catechumen a molecule of ADP to ATP and to transfer 2 electrons from the aldehyde to NAD+ to course NADH, while still releasing enough heat to the environment to brand the overall reaction energetically favorable (ΔG° for the overall reaction is -3.0 kcal/mole).
The pathway by which this remarkable feat is accomplished is outlined in Figure two-73. The chemical reactions are guided by 2 enzymes to which the carbohydrate intermediates are tightly bound. The first enzyme (glyceraldehyde three-phosphate dehydrogenase) forms a curt-lived covalent bond to the aldehyde through a reactive -SH group on the enzyme, and information technology catalyzes the oxidation of this aldehyde while still in the attached state. The high-energy enzyme-substrate bond created by the oxidation is then displaced past an inorganic phosphate ion to produce a high-free energy sugar-phosphate intermediate, which is thereby released from the enzyme. This intermediate then binds to the 2nd enzyme (phosphoglycerate kinase). This enzyme catalyzes the energetically favorable transfer of the high-energy phosphate just created to ADP, forming ATP and completing the process of oxidizing an aldehyde to a carboxylic acid (see Figure 2-73).

Figure two-73
Energy storage in steps six and 7 of glycolysis. In these steps the oxidation of an aldehyde to a carboxylic acid is coupled to the formation of ATP and NADH. (A) Step vi begins with the formation of a covalent bond between the substrate (glyceraldehyde (more...)
We have shown this particular oxidation process in some item because it provides a clear example of enzyme-mediated energy storage through coupled reactions (Effigy ii-74). These reactions (steps 6 and 7) are the only ones in glycolysis that create a loftier-energy phosphate linkage direct from inorganic phosphate. As such, they account for the net yield of two ATP molecules and two NADH molecules per molecule of glucose (encounter Panel 2-8, pp. 124–125).

Figure ii-74
Schematic view of the coupled reactions that form NADH and ATP in steps 6 and 7 of glycolysis. The C-H bond oxidation free energy drives the formation of both NADH and a high-free energy phosphate bond. The breakage of the loftier-energy bond so drives ATP formation. (more...)
As we have only seen, ATP can be formed readily from ADP when reaction intermediates are formed with college-energy phosphate bonds than those in ATP. Phosphate bonds tin be ordered in energy by comparing the standard complimentary-energy modify (ΔThousand°) for the breakage of each bail by hydrolysis. Figure 2-75 compares the high-energy phosphoanhydride bonds in ATP with other phosphate bonds, several of which are generated during glycolysis.

Effigy ii-75
Some phosphate bail energies. The transfer of a phosphate group from any molecule 1 to whatsoever molecule 2 is energetically favorable if the standard gratis-free energy change (ΔG°) for the hydrolysis of the phosphate bond in molecule 1 is more negative (more...)
Sugars and Fats Are Both Degraded to Acetyl CoA in Mitochondria
Nosotros at present move on to consider stage iii of catabolism, a process that requires arable molecular oxygen (Otwo gas). Since the Earth is idea to have developed an temper containing O2 gas betwixt 1 and two billion years agone, whereas abundant life-forms are known to have existed on the World for 3.5 billion years, the use of O2 in the reactions that we hash out next is idea to exist of relatively contempo origin. In contrast, the mechanism used to produce ATP in Figure 2-73 does not require oxygen, and relatives of this elegant pair of coupled reactions could take arisen very early on in the history of life on World.
In aerobic metabolism, the pyruvate produced by glycolysis is quickly decarboxylated by a giant circuitous of three enzymes, called the pyruvate dehydrogenase circuitous. The products of pyruvate decarboxylation are a molecule of CO2 (a waste product), a molecule of NADH, and acetyl CoA. The 3-enzyme complex is located in the mitochondria of eucaryotic cells; its structure and way of action are outlined in Figure 2-76.

Effigy ii-76
The oxidation of pyruvate to acetyl CoA and CO2. (A) The structure of the pyruvate dehydrogenase complex, which contains 60 polypeptide chains. This is an example of a large multienzyme complex in which reaction intermediates are passed directly from (more than...)
The enzymes that degrade the fatty acids derived from fats too produce acetyl CoA in mitochondria. Each molecule of fatty acid (equally the activated molecule fat acyl CoA) is cleaved downwards completely by a cycle of reactions that trims 2 carbons at a time from its carboxyl end, generating ane molecule of acetyl CoA for each plough of the bicycle. A molecule of NADH and a molecule of FADHii are also produced in this procedure (Figure two-77).

Effigy 2-77
The oxidation of fatty acids to acetyl CoA. (A) Electron micrograph of a lipid droplet in the cytoplasm (top), and the structure of fats (bottom). Fats are triacylglycerols. The glycerol portion, to which three fatty acids are linked through ester bonds, (more...)
Sugars and fats provide the major free energy sources for almost non-photosynthetic organisms, including humans. Notwithstanding, the majority of the useful energy that can be extracted from the oxidation of both types of foodstuffs remains stored in the acetyl CoA molecules that are produced by the two types of reactions only described. The citric acid cycle of reactions, in which the acetyl group in acetyl CoA is oxidized to COii and H2O, is therefore fundamental to the energy metabolism of aerobic organisms. In eucaryotes these reactions all take place in mitochondria, the organelle to which pyruvate and fat acids are directed for acetyl CoA product (Figure 2-78). Nosotros should therefore not exist surprised to discover that the mitochondrion is the place where most of the ATP is produced in fauna cells. In contrast, aerobic bacteria acquit out all of their reactions in a unmarried compartment, the cytosol, and it is here that the citric acrid bike takes identify in these cells.

Figure 2-78
Pathways for the product of acetyl CoA from sugars and fats. The mitochondrion in eucaryotic cells is the identify where acetyl CoA is produced from both types of major food molecules. It is therefore the identify where nigh of the cell's oxidation reactions (more...)
The Citric Acrid Bike Generates NADH by Oxidizing Acetyl Groups to COii
In the nineteenth century, biologists noticed that in the absence of air (anaerobic weather) cells produce lactic acid (for example, in musculus) or ethanol (for example, in yeast), while in its presence (aerobic weather) they consume Oii and produce COtwo and H2O. Intensive efforts to define the pathways of aerobic metabolism somewhen focused on the oxidation of pyruvate and led in 1937 to the discovery of the citric acid bicycle, also known as the tricarboxylic acid cycle or the Krebs cycle. The citric acrid cycle accounts for virtually two-thirds of the total oxidation of carbon compounds in almost cells, and its major end products are CO2 and high-energy electrons in the class of NADH. The CO2 is released as a waste matter product, while the high-energy electrons from NADH are passed to a membrane-bound electron-transport chain, eventually combining with Otwo to produce H2O. Although the citric acid cycle itself does not utilize Otwo, it requires O2 in order to proceed considering there is no other efficient way for the NADH to get rid of its electrons and thus regenerate the NAD+ that is needed to proceed the cycle going.
The citric acid cycle, which takes place inside mitochondria in eucaryotic cells, results in the complete oxidation of the carbon atoms of the acetyl groups in acetyl CoA, converting them into COtwo. But the acetyl grouping is non oxidized directly. Instead, this grouping is transferred from acetyl CoA to a larger, four-carbon molecule, oxaloacetate, to form the six-carbon tricarboxylic acid, citric acid, for which the subsequent bike of reactions is named. The citric acrid molecule is then gradually oxidized, assuasive the energy of this oxidation to be harnessed to produce energy-rich activated carrier molecules. The chain of 8 reactions forms a cycle considering at the end the oxaloacetate is regenerated and enters a new turn of the cycle, as shown in outline in Figure 2-79.

Effigy two-79
Elementary overview of the citric acid bike. The reaction of acetyl CoA with oxaloacetate starts the cycle past producing citrate (citric acid). In each turn of the wheel, ii molecules of COii are produced equally waste products, plus three molecules of NADH, one (more...)
Nosotros have thus far discussed only ane of the three types of activated carrier molecules that are produced by the citric acrid wheel, the NAD+-NADH pair (see Effigy 2-sixty). In addition to three molecules of NADH, each turn of the cycle also produces one molecule of FADH two (reduced flavin adenine dinucleotide) from FAD and i molecule of the ribonucleotide GTP (guanosine triphosphate) from GDP. The structures of these two activated carrier molecules are illustrated in Figure 2-80. GTP is a close relative of ATP, and the transfer of its final phosphate group to ADP produces one ATP molecule in each cycle. Like NADH, FADHtwo is a carrier of high-energy electrons and hydrogen. As we discuss shortly, the energy that is stored in the readily transferred high-energy electrons of NADH and FADHii will be utilized subsequently for ATP production through the process of oxidative phosphorylation, the but step in the oxidative catabolism of foodstuffs that directly requires gaseous oxygen (O2) from the atmosphere.
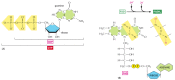
Effigy ii-80
The structures of GTP and FADH2. (A) GTP and GDP are close relatives of ATP and ADP, respectively. (B) FADH2 is a carrier of hydrogens and high-energy electrons, like NADH and NADPH. It is shown here in its oxidized form (FAD) with the hydrogen-carrying (more...)
The complete citric acid cycle is presented in Console 2-9 (pp. 126–127). The actress oxygen atoms required to make CO2 from the acetyl groups inbound the citric acid cycle are supplied non past molecular oxygen, but by water. As illustrated in the panel, three molecules of water are split in each cycle, and the oxygen atoms of some of them are ultimately used to make COtwo.
![]()
In add-on to pyruvate and fatty acids, some amino acids pass from the cytosol into mitochondria, where they are also converted into acetyl CoA or one of the other intermediates of the citric acid cycle. Thus, in the eucaryotic prison cell, the mitochondrion is the center toward which all energy-yielding processes pb, whether they begin with sugars, fats, or proteins.
The citric acid cycle besides functions equally a starting point for important biosynthetic reactions by producing vital carbon-containing intermediates, such as oxaloacetate and α-ketoglutarate. Some of these substances produced by catabolism are transferred back from the mitochondrion to the cytosol, where they serve in anabolic reactions as precursors for the synthesis of many essential molecules, such equally amino acids.
Electron Transport Drives the Synthesis of the Majority of the ATP in Most Cells
It is in the last pace in the degradation of a food molecule that the major portion of its chemic energy is released. In this last process the electron carriers NADH and FADH2 transfer the electrons that they take gained when oxidizing other molecules to the electron-transport chain, which is embedded in the inner membrane of the mitochondrion. As the electrons pass forth this long chain of specialized electron acceptor and donor molecules, they autumn to successively lower energy states. The energy that the electrons release in this process is used to pump H+ ions (protons) beyond the membrane—from the inner mitochondrial compartment to the exterior (Figure 2-81). A slope of H+ ions is thereby generated. This slope serves as a source of energy, beingness tapped like a bombardment to drive a variety of free energy-requiring reactions. The most prominent of these reactions is the generation of ATP by the phosphorylation of ADP.

Figure 2-81
The generation of an H+ gradient across a membrane by electron-send reactions. A loftier-energy electron (derived, for example, from the oxidation of a metabolite) is passed sequentially by carriers A, B, and C to a lower energy state. In this diagram (more...)
At the end of this series of electron transfers, the electrons are passed to molecules of oxygen gas (Oii) that have diffused into the mitochondrion, which simultaneously combine with protons (H+) from the surrounding solution to produce molecules of h2o. The electrons have now reached their lowest free energy level, and therefore all the available energy has been extracted from the nutrient molecule being oxidized. This procedure, termed oxidative phosphorylation (Figure 2-82), as well occurs in the plasma membrane of bacteria. As one of the most remarkable achievements of cellular evolution, information technology will exist a fundamental topic of Chapter 14.
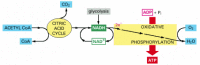
Figure 2-82
The terminal stages of oxidation of food molecules. Molecules of NADH and FADH2 (FADH2 is not shown) are produced by the citric acrid cycle. These activated carriers donate high-free energy electrons that are somewhen used to reduce oxygen gas to water. A major (more than...)
In full, the complete oxidation of a molecule of glucose to H2O and CO2 is used past the jail cell to produce nearly xxx molecules of ATP. In contrast, just ii molecules of ATP are produced per molecule of glucose past glycolysis lonely.
Organisms Store Food Molecules in Special Reservoirs
All organisms need to maintain a high ATP/ADP ratio, if biological social club is to be maintained in their cells. Nonetheless animals take only periodic admission to food, and plants need to survive overnight without sunlight, without the possibility of sugar production from photosynthesis. For this reason, both plants and animals catechumen sugars and fats to special forms for storage (Figure 2-83).

Effigy ii-83
The storage of sugars and fats in brute and institute cells. (A) The structures of starch and glycogen, the storage form of sugars in plants and animals, respectively. Both are storage polymers of the sugar glucose and differ only in the frequency of co-operative (more than...)
To compensate for long periods of fasting, animals store fatty acids as fat aerosol composed of water-insoluble triacylglycerols, largely in specialized fat cells. And for shorter-term storage, carbohydrate is stored as glucose subunits in the big branched polysaccharide glycogen, which is present as pocket-size granules in the cytoplasm of many cells, including liver and muscle. The synthesis and degradation of glycogen are chop-chop regulated co-ordinate to need. When more ATP is needed than tin can be generated from the food molecules taken in from the bloodstream, cells break down glycogen in a reaction that produces glucose one-phosphate, which enters glycolysis.
Quantitatively, fat is a far more important storage form than glycogen, in part because the oxidation of a gram of fatty releases about twice as much free energy as the oxidation of a gram of glycogen. Moreover, glycogen differs from fatty in binding a smashing deal of h2o, producing a sixfold departure in the actual mass of glycogen required to shop the same amount of energy as fat. An boilerplate adult man stores enough glycogen for just nearly a day of normal activities but enough fat to concluding for most a month. If our main fuel reservoir had to be carried every bit glycogen instead of fatty, torso weight would need to be increased by an average of about sixty pounds.
Most of our fat is stored in adipose tissue, from which it is released into the bloodstream for other cells to use as needed. The demand arises after a period of non eating; even a normal overnight fast results in the mobilization of fatty, so that in the morning time most of the acetyl CoA entering the citric acrid cycle is derived from fatty acids rather than from glucose. Afterward a meal, nevertheless, most of the acetyl CoA inbound the citric acid bike comes from glucose derived from food, and any excess glucose is used to replenish depleted glycogen stores or to synthesize fats. (While animal cells readily convert sugars to fats, they cannot convert fat acids to sugars.)
Although plants produce NADPH and ATP by photosynthesis, this important procedure occurs in a specialized organelle, chosen a chloroplast, which is isolated from the rest of the plant cell by a membrane that is impermeable to both types of activated carrier molecules. Moreover, the plant contains many other cells—such as those in the roots—that lack chloroplasts and therefore cannot produce their own sugars or ATP. Therefore, for most of its ATP product, the establish relies on an export of sugars from its chloroplasts to the mitochondria that are located in all cells of the plant. Most of the ATP needed past the plant is synthesized in these mitochondria and exported from them to the rest of the establish jail cell, using exactly the same pathways for the oxidative breakdown of sugars that are utilized by nonphotosynthetic organisms (Figure 2-84).

Figure 2-84
How the ATP needed for most plant cell metabolism is made. In plants, the chloroplasts and mitochondria collaborate to supply cells with metabolites and ATP.
During periods of excess photosynthetic capacity during the day, chloroplasts convert some of the sugars that they make into fats and into starch, a polymer of glucose coordinating to the glycogen of animals. The fats in plants are triacylglycerols, just like the fats in animals, and differ only in the types of fatty acids that predominate. Fatty and starch are both stored in the chloroplast equally reservoirs to be mobilized equally an free energy source during periods of darkness (meet Figure 2-83B).
The embryos inside plant seeds must live on stored sources of energy for a prolonged menstruum, until they germinate to produce leaves that can harvest the free energy in sunlight. For this reason found seeds often contain specially large amounts of fats and starch—which makes them a major food source for animals, including ourselves (Figure 2-85).

Figure two-85
Some plant seeds that serve every bit important foods for humans. Corn, nuts, and peas all contain rich stores of starch and fat that provide the young plant embryo in the seed with free energy and building blocks for biosynthesis. (Courtesy of the John Innes Foundation.) (more than...)
Amino Acids and Nucleotides Are Part of the Nitrogen Bicycle
In our word so far we have concentrated mainly on carbohydrate metabolism. We have not yet considered the metabolism of nitrogen or sulfur. These 2 elements are constituents of proteins and nucleic acids, which are the two near important classes of macromolecules in the cell and make up approximately 2-thirds of its dry weight. Atoms of nitrogen and sulfur pass from compound to compound and betwixt organisms and their environs in a serial of reversible cycles.
Although molecular nitrogen is abundant in the Earth's atmosphere, nitrogen is chemically unreactive equally a gas. Only a few living species are able to incorporate it into organic molecules, a process chosen nitrogen fixation. Nitrogen fixation occurs in sure microorganisms and by some geophysical processes, such as lightning discharge. It is essential to the biosphere as a whole, for without it life would non exist on this planet. Only a small fraction of the nitrogenous compounds in today's organisms, however, is due to fresh products of nitrogen fixation from the temper. Almost organic nitrogen has been in circulation for some time, passing from one living organism to another. Thus present-twenty-four hour period nitrogen-fixing reactions can be said to perform a "topping-upward" function for the total nitrogen supply.
Vertebrates receive virtually all of their nitrogen in their dietary intake of proteins and nucleic acids. In the torso these macromolecules are cleaved downwardly to amino acids and the components of nucleotides, and the nitrogen they incorporate is used to produce new proteins and nucleic acids or utilized to make other molecules. About one-half of the 20 amino acids establish in proteins are essential amino acids for vertebrates (Figure two-86), which means that they cannot be synthesized from other ingredients of the diet. The others can be so synthesized, using a diversity of raw materials, including intermediates of the citric acid cycle as described below. The essential amino acids are made by nonvertebrate organisms, usually by long and energetically expensive pathways that have been lost in the course of vertebrate evolution.

Figure two-86
The nine essential amino acids. These cannot be synthesized by human cells and then must exist supplied in the nutrition.
The nucleotides needed to brand RNA and DNA tin exist synthesized using specialized biosynthetic pathways: at that place are no "essential nucleotides" that must exist provided in the nutrition. All of the nitrogens in the purine and pyrimidine bases (equally well as some of the carbons) are derived from the plentiful amino acids glutamine, aspartic acrid, and glycine, whereas the ribose and deoxyribose sugars are derived from glucose.
Amino acids that are not utilized in biosynthesis can be oxidized to generate metabolic energy. Near of their carbon and hydrogen atoms somewhen form COii or H2O, whereas their nitrogen atoms are shuttled through various forms and eventually appear as urea, which is excreted. Each amino acid is processed differently, and a whole constellation of enzymatic reactions exists for their catabolism.
Many Biosynthetic Pathways Brainstorm with Glycolysis or the Citric Acid Wheel
Catabolism produces both free energy for the cell and the building blocks from which many other molecules of the jail cell are made (meet Figure 2-36). Thus far, our discussions of glycolysis and the citric acid cycle have emphasized energy production, rather than the provision of the starting materials for biosynthesis. Only many of the intermediates formed in these reaction pathways are also siphoned off by other enzymes that use them to produce the amino acids, nucleotides, lipids, and other pocket-sized organic molecules that the prison cell needs. Some idea of the complication of this process tin be gathered from Figure ii-87, which illustrates some of the branches from the key catabolic reactions that atomic number 82 to biosyntheses.
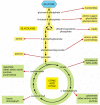
Figure 2-87
Glycolysis and the citric acid cycle provide the precursors needed to synthesize many important biological molecules. The amino acids, nucleotides, lipids, sugars, and other molecules—shown here as products—in turn serve as the precursors (more...)
The existence of so many branching pathways in the cell requires that the choices at each branch be advisedly regulated, as nosotros hash out next.
Metabolism Is Organized and Regulated
I gets a sense of the intricacy of a jail cell as a chemic machine from the relation of glycolysis and the citric acid cycle to the other metabolic pathways sketched out in Figure two-88. This type of chart, which was used before in this chapter to introduce metabolism, represents only some of the enzymatic pathways in a jail cell. It is obvious that our give-and-take of cell metabolism has dealt with only a tiny fraction of cellular chemistry.

Figure 2-88
Glycolysis and the citric acid cycle are at the center of metabolism. Some 500 metabolic reactions of a typical cell are shown schematically with the reactions of glycolysis and the citric acrid cycle in red. Other reactions either atomic number 82 into these two (more...)
All these reactions occur in a cell that is less than 0.1 mm in diameter, and each requires a unlike enzyme. As is clear from Figure 2-88, the same molecule tin can often be part of many unlike pathways. Pyruvate, for example, is a substrate for half a dozen or more different enzymes, each of which modifies it chemically in a different way. One enzyme converts pyruvate to acetyl CoA, another to oxaloacetate; a third enzyme changes pyruvate to the amino acid alanine, a 4th to lactate, and so on. All of these different pathways compete for the aforementioned pyruvate molecule, and similar competitions for thousands of other small-scale molecules go on at the same time. A better sense of this complexity can mayhap be attained from a three-dimensional metabolic map that allows the connections between pathways to be made more directly (Figure 2-89).
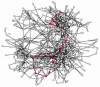
Figure two-89
A representation of all of the known metabolic reactions involving minor molecules in a yeast cell. As in Figure ii-88, the reactions of glycolysis and the citric acid cycle are highlighted in red. This metabolic map is unusual in making use of three-dimensions, (more than...)
The situation is further complicated in a multicellular organism. Different prison cell types will in general crave somewhat different sets of enzymes. And different tissues make distinct contributions to the chemistry of the organism as a whole. In add-on to differences in specialized products such as hormones or antibodies, in that location are significant differences in the "common" metabolic pathways amongst various types of cells in the aforementioned organism.
Although nearly all cells comprise the enzymes of glycolysis, the citric acid cycle, lipid synthesis and breakdown, and amino acrid metabolism, the levels of these processes required in different tissues are not the same. For example, nerve cells, which are probably the near fastidious cells in the body, maintain almost no reserves of glycogen or fatty acids and rely almost entirely on a constant supply of glucose from the bloodstream. In dissimilarity, liver cells supply glucose to actively contracting muscle cells and recycle the lactic acid produced past musculus cells back into glucose (Figure 2-90). All types of cells have their distinctive metabolic traits, and they cooperate extensively in the normal state, likewise as in response to stress and starvation. 1 might think that the whole system would need to be so finely balanced that any minor upset, such as a temporary change in dietary intake, would be disastrous.
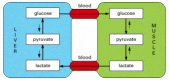
Effigy 2-90
Schematic view of the metabolic cooperation betwixt liver and musculus cells. The master fuel of actively contracting muscle cells is glucose, much of which is supplied past liver cells. Lactic acid, the cease production of anaerobic glucose breakdown by glycolysis (more...)
In fact, the metabolic residue of a cell is amazingly stable. Whenever the rest is perturbed, the jail cell reacts and so as to restore the initial country. The cell can suit and continue to function during starvation or disease. Mutations of many kinds can damage or even eliminate particular reaction pathways, and notwithstanding—provided that certain minimum requirements are met—the cell survives. It does so considering an elaborate network of control mechanisms regulates and coordinates the rates of all of its reactions. These controls rest, ultimately, on the remarkable abilities of proteins to alter their shape and their chemical science in response to changes in their immediate environment. The principles that underlie how large molecules such every bit proteins are built and the chemical science behind their regulation will be our next concern.
Summary
Glucose and other food molecules are broken down by controlled stepwise oxidation to provide chemic energy in the course of ATP and NADH. These are three main sets of reactions that act in series—the products of each being the starting material for the next: glycolysis (which occurs in the cytosol), the citric acrid cycle (in the mitochondrial matrix), and oxidative phosphorylation (on the inner mitochondrial membrane). The intermediate products of glycolysis and the citric acid cycle are used both as sources of metabolic energy and to produce many of the small molecules used as the raw materials for biosynthesis. Cells store sugar molecules equally glycogen in animals and starch in plants; both plants and animals also use fats extensively as a nutrient shop. These storage materials in turn serve every bit a major source of food for humans, along with the proteins that comprise the majority of the dry mass of the cells we eat.
Source: https://www.ncbi.nlm.nih.gov/books/NBK26882/
0 Response to "What would happen to the supply of ATP in your cells if you did not eat enough carbohydrates"
Post a Comment